Pflugers Arch. 2003 May;446(2):148-53. Epub 2003 Feb 15.
Source
Laboratorium voor Fysiologie, K.U. Leuven Campus Gasthuisberg, Herestraat 49, 3000, Leuven, Belgium. frank.wuytack@med.kuleuven.ac.be
Abstract
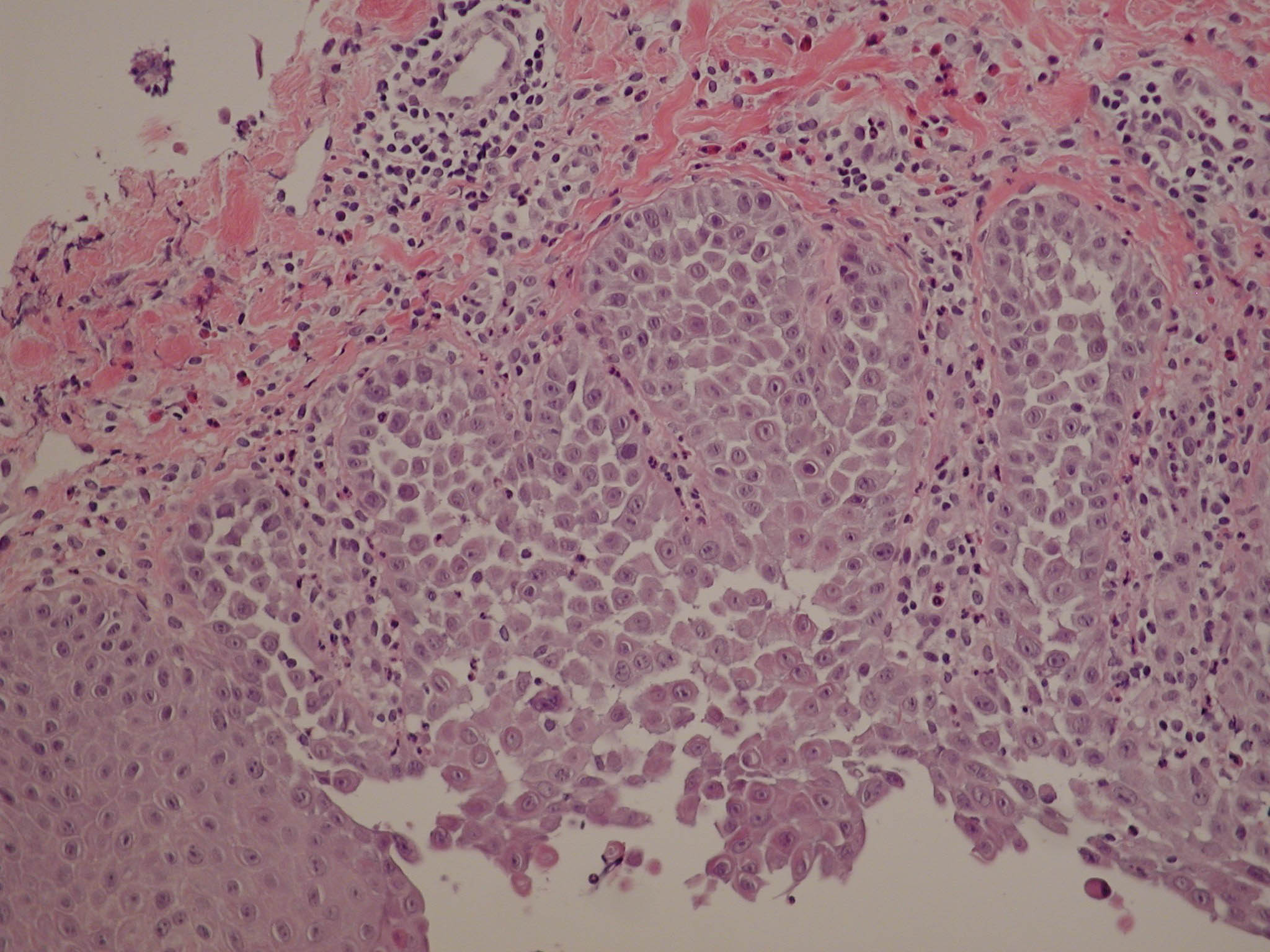
Besides the well-known sarco/endoplasmic-reticulum Ca(2+)-transport ATPases (SERCA), animal cells contain a much less characterized P-type Ca(2+)-transport ATPase: the PMR1/SPCA Ca(2+)/Mn(2+)-transport ATPase. SPCA is mainly targeted to the Golgi apparatus. Phylogenetic analysis indicates that it might be more closely related to a putative ancestral Ca(2+) pump than SERCA. SPCA supplies the Golgi apparatus, and possibly other more distal compartments of the secretory pathway, with the Ca(2+) and Mn(2+) necessary for the production and processing of secretory proteins. In the lactating mammary gland, SPCA appears to be the primary pump responsible for supplementing the milk with high (60-100 mM) Ca(2+). It could also play a role in detoxification of cells overloaded with Mn(2+). Mutations in the human gene encoding the SPCA pump ( ATP2C1) result in Hailey-Hailey disease, a keratinocyte disorder characterized by incomplete cell adhesion. Recent observations show that the Golgi apparatus can function as a Ca(2+) store, which can be involved in setting up cytosolic Ca(2+) oscillations.